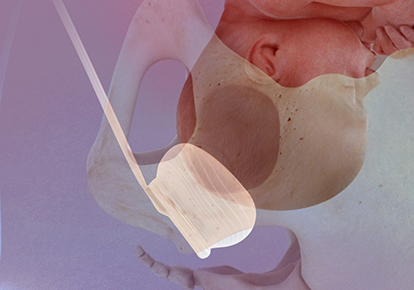
Fetal Pillow is intended to elevate the fetal head and facilitate delivery of the fetus in women requiring a Cesarean section at full dilation or those requiring a Cesarean section after a failed instrumental vaginal delivery. Fetal Pillow is indicated for use in gestational age >37 weeks.
Fetal Pillow Balloon Cephalic Elevation Device for Cesarean Sections
Product codes
| Product | Order Code |
|---|---|
| Fetal Pillow (6/Box) | FP-010 |
Get In Touch With Us
We'd love to hear from you. How can we help?
Brochures, Catalogs & Flyers
Product Brochures
Product Brochures
Product Catalogs
Product Catalogs
Related Products

WarmGel® Infant Heel Warmers
A disposable heel warmer for use when circulation needs to be stimulated in the newborn or infant heel in order...

The Mystic II® Vacuum-Assisted Systems for Deliveries
Vacuum-Assisted system designed to assist a practitioner in the delivery of an infant during childbirth.

TransWarmer® Infant Transport Mattress
Provides warmth during transport of infant within the hospital or between hospitals.
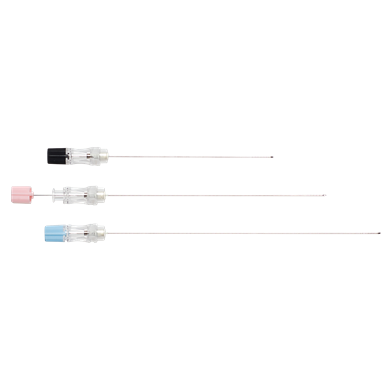
Wallace® Amniocentesis Needles
Sterile, single-use, devices for the removal of amniotic fluid, trans-abdominally under ultrasound guidance.
Webinars
Articles
Support & Compliance
Our global team is committed to providing the highest standards of service and support.

Batch Certificates
Use this tool to enter your batch number and download the corresponding certificate of analysis.

Service
We offer a range of contract options to suit your needs: preventative maintenance and service, reliable access to spare parts, product training, and online handling of service requests.
Get In Touch With Us
We'd love to hear from you. How can we help?
